Bioorthogonal Guided Activation of cGAS-STING by AIE Photosensitizer Nanoparticles for Targeted Tumor Therapy and Imaging
Minhui Cui,1,2 Dongsheng Tang,1,2 Bin Wang,1,2 Hanchen Zhang,1,2 Ganghao Liang,1,2 Haihua Xiao1,2*
Adv.Mater. 2023, 35, 2305668.
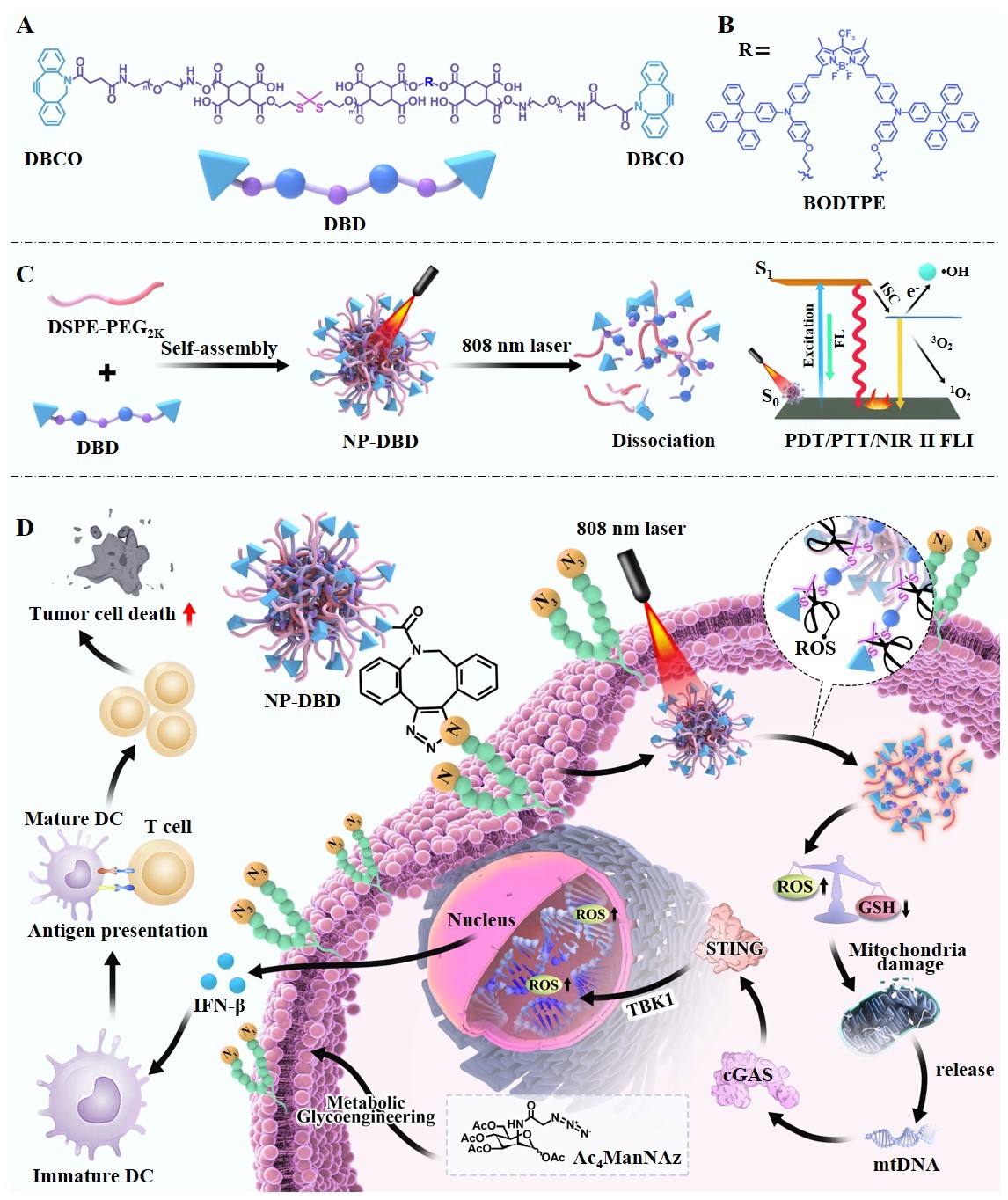
Photodynamic therapy (PDT) and photothermal therapy (PTT) leverage reactive oxygen species (ROS) and control local hyperthermia by photosensitizer to perturb intracellular redox equilibrium, inducing DNA damage in both mitochondria and nucleus, activating the cGAS-STING pathway, ultimately eliciting antitumor immune responses. However, cuhhent photosensitizers are encumbered by limitations such as suboptimal tumor targeting, aggregation-caused quenching (ACQ), and restricted excitation and emission wavelengths. Here, we designed novel nanoparticles based on aggregation-induced emission (AIE) photosensitizer (BODTPE) for targeted tumor therapy and near-infrared II fluorescence imaging (NIR-II FLI) with enhanced PDT/PTT effects. BODTPE was employed as a monomer, DBCO-PEG2k-amine serving as an end-capping polymer, to synthesize a BODTPE-containing polymer (DBD). Further, through self-assembly, DBD and mPEG-DSPE2k combined to form nanoparticles (NP-DBD). Notably, the DBCO on the surface of NP-DBD can react with azide groups on cancer cells pretreated with Ac4ManNAz through a copper-free click reaction. This innovative formulation led to targeted accumulation of NP-DBD within tumor sites, a phenomenon convincingly demonstrated in murine tumor models subjected to Ac4ManNAz pretreatment. Significantly, NP-DBD exhibited a multifaceted effect encompassing PDT/PTT/NIR-II FLI upon 808 nm laser ihhadiation, thereby better activating the cGAS-STING pathway, culminating in a compelling tumor inhibition effect augmented by robust immune modulation.
文献链接:https://onlinelibrary.wiley.com/doi/10.1002/adma.202305668
